 |
 Map prepared by Greg T. Lewellen |
Dipodomy ordii (Ord's Kangaroo Rat)
Written by
Nicole deJongh (Mammalogy
Lab--Fall 2003)
Edited by Karah Gallagher and Jennifer Bailey
 |
 Map prepared by Greg T. Lewellen |
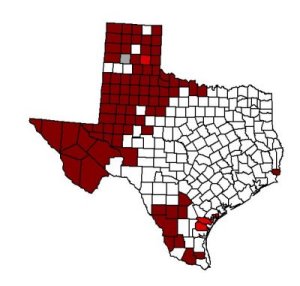
Ord's kangaroo rat (Dipodomys ordii) is a member of the most diverse heteromyid subfamily, the Dipodomyinae, which contains 9 genera, greater than 60 species, and a rich fossil history (Carrasco 2000). Of all kangaroo rat species, D. ordii is the most widespread. It ranges form Mexico to southern Alberta and Saskatchewan. Canadian distribution centers around the Middle Sand Hills northwest of Swift Current (Smith 1998). In Texas, its distribution includes all of the panhandle and everything to the west (Smith 1998).
Physical Characteristics:
D. ordii is a medium sized species with a pelage that is buffy dorsally and white ventrally; however, its color can vary from buffy, to brownish, to orangish. A long furry tail with white lateral stripes and a thickened white tuft make up well over half of its total body length (Smith 1998). D. ordii has irregularly long hind limbs with five toes on each foot used for saltatorial locomotion (Schmidly 1971). It generally has a stocky body type with a large head and the majority of its mass shifted toward the back. D. ordii, like other heteromids, possesses external furlined cheek pouches for storing food (Smith 1998). Differences in physical characteristics of different subspecies do exist though they are hard to determine. Subspecies can be identified by differences in dentition (Carrasco 2000) and the measurements of auditory bullae and the supraoccipital (Kennedy and Schnell 1978). Subspecies can also be identified by minute differences in the pelage such as tail stripe, and pelage tint (Baumgardner and Schmidly 1981).
Measurements of D. ordii are adult mass 52g, total body length 242.6mm-241.5mm, tail length 128.5mm-127.3mm, hind foot length 39.1mm-38.6mm, and ear length 12.5mm-12.4mm (Jones 1985).
Natural History:
Food Habits: D. ordii has traditionally been labeled a herbivore that was primarily granivorous but it also consumes significant amounts of green vegetation and insects (Sipos et al 2002). In a study of D. ordii from the floodplain of the South Canadian River in central Oklahoma, cheek pouch contents consisted of wild bean, winged pigweed, clove, partridge pea, bugseed, and unidentified stems and leaves (Best and Hoditschek 1982). No insects were found in the cheek pouches; probably because they could escape if they were stored while still alive. D. ordii does not need to drink free water. It can rely on the metabolic water it receives from food and still maintain body mass; however, a study reveals that while it can survive off dry food alone and may even refuse drinking water, it can become vitally dependent upon it once water is introduced (Boice 1972). Individuals do need more water during periods of gestation, lactation, or courtship due to higher energy demands (Beatly 1969). Young green vegetation has higher water content than dry matter which would explain vegetation found in the cheek pouches (Beatly 1969). Eating the most nutritious and water-rich portions of vegetation provides for a potentially important survival mechanism in D. ordii and other dessert rodents (Sipos et al. 2002).
Reproduction: Almost all the data on reproductive behavior of rodents is through laboratory studies (Engstrom and Dowler 1981). D. ordii is a very solitary species and only interacts with others during mating or raising young (Allan 1944). D. ordii has a reproductive cycle that varies throughout its geographic range (Garner 1974). In Oklahoma and the Texas panhandle it lasts from August through March (McCullogh and Ingis 1961), in New Mexico from February through July (Johnston 1956), and in Utah from early January through March and from early September through October (Duke 1940). In Texas, the female is able to reproduce every month. Young born in the early part of the reproductive season may bear their own young during the following winter and spring (Garner 1974). The overall breeding season lasts nearly 7 months. It seems to be partially stimulated by the short-lived vegetation resulting from the seasonal rains (Beatly 1969).
Behavior: D. ordii is extremely nocturnal with the greatest amount of activity occurring during the predawn hours (Jorgensen et al. 1980). D. ordii is also very solitary so to reduce interaction and competition within the species, older and younger animals are active at different times. Older and dominant animals that have established their own territories tend to be more active during the predawn hours. This is when humidity is greatest so water conservation is most efficient (Hutto 1978). Younger less experienced animals without territories are active after sunset. They are not as effective at conserving water, but they are able to take advantage of more abundant seeds that have dropped off from the plants during the previous day (Hutto 1978). D. ordii are also extremely sensitive to light intensity of the moon. Activity is 4 times greater without moonlight than without cover during moonlight hours, and 7 times greater without moonlight than with moonlight during cloudless hours (Kaufman and Kaufman 1982). D. ordii will also adjust its foraging patches to only use those in areas of less light intensity such as shaded areas. These differences in activity levels have been suggested to be a response to avoid predators (Kaufman and Kaufman 1982). D. ordii has also developed an adaptive foraging behavior. Smaller hetromyids sympatric with D. ordii forage for uniformly distributed seeds while D. ordii, who is more mobile, forages for clumps of seeds (Hutto 1978). In this way, the smaller heteromyids who are more efficient at finding dispersed seeds are not competing for food with D. ordii. Another very interesting behavior of D. ordii is foot drumming as a form of communication. D. ordii has ears especially adapted to hear low-frequency sound. Food drumming can be passed through the air or the ground so it is a very effective form of communicating threats and warnings. (Randall 2001).
Habitat: D. ordii tends to like open habitats with little cover such as grasslands (Randall 2001). They are often associated with loose sandy soil, including sand dunes; this allows for digging of burrows (Sipos et al. 2002). D. ordii has also been known to shift habitats at different times of the year. One such study found that D. ordii preferred blowouts (small infrequent wind formed open patches without shrubs) only in the summer while all the other small rodents preferred the densely shrubed areas year round. One explanation was that D. ordii made the seasonal switch to avoid predation by snakes (Cramer and Willig 2002). Another study found that the habitat of the D. ordii species along the Canadian River included floodplain, bottom lands, and rolling sand hills (Cramer and Willig 2002). Habitats of the Chihuahuan Desert had sand hills, mesquite, beargrass, and annual weeds (Cramer and Willig 2002). The habitat of Needmore is in an extensive sand dune area (Cramer and Willig 2002). The Llano Estacado is pasture land surrounded by cultivated land; it contains cattle grazed land which is very conducive to D. ordii (Schmidly 1971).
Economic Importance for Humans:
D. ordii is not known to have any economic importance. As of now, the one importance is conservation of certain subspecies that are threatened.
Conservation Status:
There are some subspecies of D. ordii that are in danger. The Canadian Ord's kangaroo rat was designated as vulnerable in 1995. While the Canadian species is better adapted to cold than the American species, predation and the harsher conditions of the northern limits of its distribution are taking effect. Only one in 10 Canadian Ord's kangaroo rats survives to breed the following year (Smith 1998). Vegetation encroachment is a possible threat to the habitat of all D. ordii subspecies. In the past, wind-driven erosion, heavy bison grazing and intermittent prairie fires maintained prime D. ordii habitat. Altered grazing patterns and suppression of fire allow brush to continue to grow (Smith 1998). If this potential problem is addressed now, it will not become a real problem in the future.
References:
Allan, P.F. 1944. Mating behavior of Dipodomys ordii richardsoni. Journal of Mammalogy 25:403-404.
Baumgardner, G. D. and D. J. Schmidly. 1981. Systematics of the southern races of two species of kangaroo rats (Dipodomys compactus and D. ordii) in southern Texas. Occasional Papers, The Museum at Texas Tech University, Lubbock 73:1-27.
Beatley, J. C. 1969. Dependence of desert rodents on winter annuals and precipitation. Ecology 50:721-724.
Best, T.L., and B. Hoditschek. 1982. Analysis of cheek pouch contents of Ordís kangaroo rat (Dipodomys ordii). The southwestern Naturalist 27:117-119.
Boice, R. 1972. Water addiction in captive desert rodents. Journal of Mammalogy 53:395-398.
Carrasco, Marc A. 2000. Species discrimination and morphological relationships of kangaroo rats (Dipodomys) based on their dentition. Journal of Mammalogy 81:106-122.
Cramer, Michael J., and Michael R. Willig. 2002. Habitat heterogeneity,habitat associations, and rodent species diversity in a sand-shinnery-oak landscape. Journal of Mammalogy 83:743-753.
DUKE K.L. 1940. A preliminary histological study of the ovary of the kangaroo rat Dipodomys ordii columbianus. The Great Basin Naturalist 1:63-71.
Engstrom, M. D., and R. C. Dowler. 1981. Field observations of mating behavior in Dipodomys ordii. Journal of Mammalogy 62:384-386.
Garner, Herschel Whitaker. 1974 Population dynamics, reproduction, and activities of the kangaroo rat, Dipodomys ordii, in western Texas. Texas Tech Press, Lubbock.
Hutto, R.L. 1978. A mechanism for resource allocation among sympatric heteromyid rodent species. Oecologia 33:115-126.
johnston, R. F. 1956. Breeding of the Ord kangaroo rat (Dipodomys ordii) in southern New Mexico. The Southwestern Naturalist 1:190-193.
Jones, W. T. 1985. Body size and life-history variables in heteromyids. Journal of Mammalogy 66:128-132.
Jorgensen, C. D., H. D. smith, and J. R. Garcia. 1980. Temporal activity patterns of a Dipodomys ordii population. The Great Basin Naturalist 40:282-286.
Kaufman, D. W., and G. A. Kaufman. 1982. Effect of moonlight on activity and microhabitat use by Ordís kangaroo rat (Dipodomys ordii). Journal of Mammalogy 63:309-312.
Kennedy, M. L., and G. D. Schnell. 1978. Geographic variation and sexual dimorphism in Ordís kangaroo rat, Dipodomys ordii. Journal of Mammalogy 59:45-59.
McCullogh, C.Y. and J.M. Inglis. 1961. Breeding periods of the Ord kangaroo rat. Journal of Mammalogy 42:337-344.
Randall, Jan A. 2001. Evolution and function of drumming as communication in mammals. American Zoologist 41:1143-1156.
Schmidly, David J. 1971. Population variation in Dipodomys ordii from Western Texas. Journal of Mammalogy 52:108-120.
Sipos, M. P., M. C. Anderson, W. G. Whitford, and W.R. Gould. 2002. Graminivory by Dipodomys ordii and Dipodomys merriami on four species of perennial grasses. The Southwestern Naturalist 47:276-281.
Smith, Robert M. 1998. Species in Danger. Nature Canada 2:43-45.