 |
 Map prepared by Greg T. Lewellen |
Lasiurus seminolus (Seminole Bat)
Written by
Theresa Albrecht (Mammalogy
Lab--Fall 2003)
Edited by Karah Gallagher and Jennifer Bailey
 |
 Map prepared by Greg T. Lewellen |
Lasiurus seminolus was named after the Seminole Indians, who inhabited the region from which the bat was first known (Lowery 1974).
The range of Lasiurus seminolus includes the southeastern states along the Gulf of Mexico and along the southern Atlantic coast. The most western location begins in the eastern portion of Texas and extends eastward across Louisiana, Mississippi, Alabama, and Georgia, also south into southern Florida. The Seminole batís range extends as far south as northeastern Mexico (Barbour and Davis 1969; Lowery 1974). Its range extends northward to the coastal and Piedmont regions of North and South Carolina (Lowery 1974). Records of Lasiurus seminolus beyond North Carolina have been in southeastern Pennsylvania. The first record was in Berks County, PA (Poole 1932). The second record was in Lancaster County, PA (Poole 1949). An additional record for the Seminole bat exists in Tompkins County, New York (Layne 1955).
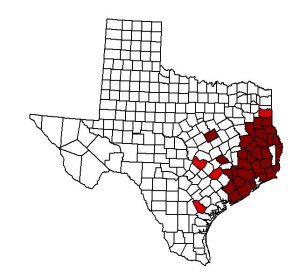
East Texas is broken up into two regions: the eastern half of the Texan Province and the western extremities of the Austroriparian Biotic Province. The seminole bat can be found in both biotic regions. The northernmost counties are Rusk and Panola, while the southern most county is Matagorda (Schmidly et al. 1977). This species reaches the western limits of its distribution in the United States within the bounds of eastern Texas (Schmidly 1983). The most western site is Burleson County, with the exception of McLennan County, which lies west of the East Texas boundary line. The most eastern point of Texas where Lasiurus seminolus may be found is in Newton County, which lies along the Texas-Louisiana border (Schmidly et al. 1977).
Physical Characteristics:
Lasiurus seminolus is a medium-sized bat, with general characters similar to that of Lasiurus borealis (red bat). Its full and soft fur is a rich mahogany brown with the back slightly frosted with gray (Barbour and Davis 1969). Fur covering the throat, chest, and front of the shoulder vary with whitish shades. The muzzle, backs of ears, and fur bordering the forearm is yellowish brown (Miller 1897). Patches of hair can be seen at the base of the thumb, third, fourth, and fifth fingers (Lowery 1974). On the middle of the back, the fur is about 12 millimeters in length. In this region the individual hairs are arranged in four bands as follows: basal band, deep blackish plumbeous; middle band, light gray; subapical band, rich mahogany; and extreme tip, grayish white (Miller 1897). A major distinction of the seminole bat from the red bat is the light frosting of the fur produced by the white tips of the hairs on the dorsum (Lowery 1974; Schmidly 1983). Pelage of the specimen from Tompkins County, New York was somewhat more frosted on the dorsum than the average Seminole bat of the more southern Atlantic states (Layne 1955).
The ears are short and rounded with a triangular shaped tragus (Barbour and Davis 1969; Lowery 1974; Miller 1897). The flight membranes are attached at the base of the toes, and the tail is contained entirely within the uropatagium (Miller 1897). The dorsal side of the interfemoral membrane is thickly covered with fur (Lowery 1974; Miller 1897). Lasiurus seminolus has a calcar, which is about twice as long as its small feet (Miller 1897).
Average external measurements are: total length 99mm, tail 43mm, hindfoot 7mm, and ear 11mm (Schmidly 1983). Measurements of male specimens from Louisiana averaged: total length 97.7mm, tail 39.7mm, hindfoot 8.3mm, ear length 9.0mm, and forearm length 39.7mm. Measurements of female specimens from Louisiana averaged: total length 103.5mm, tail 45.5mm, hindfoot 8.2mm, and forearm length 40.7mm. The above measurements indicate that females are slightly larger than males (Lowry 1974). The dimensions of the specimen from Berks County, Pennsylvania are: total length 112mm, tail 48mm, and hindfoot 10mm (Poole 1932). The dimensions of the specimen from Tompkins County, New York are: total length 110mm, tail 53mm, hindfoot 9mm, forearm 42mm, and weight 13.0 grams (Layne 1955).
The Seminole bat is similar to the red bat in terms of the skull and dentition. A distinguishing factor on the skull of Lasiurus seminolus is the presence of a more pronounced ridge above the lacrimals (Lowery 1974; Schmidly 1983). The skull is described as broad and robust (Hall 1981). Lengths of the zygomatic breadths from specimens in Louisiana are as follows: males 9.2mm and females 9.6mm (Lowery 1974).
The dental formula of Lasiurus seminolus is i 1/3 c 1/1 p 2/2 m 3/3 with a total of 32 teeth (Hall 1981; Miller 1897).
Natural History:
Food Habits: Lasiurus seminolus is an insectivorous bat. This bat forages at treetop level, about 7 to 15 meters above ground for flying insects (Barbour and Davis 1969; Sherman 1939). This species had been known to feed around streetlights (Jennings 1958). The seminole bat feeds on three major orders of insects, Homoptera, Diptera, and Coleoptera (Barbour and Davis 1969; Sherman 1939). At a river swamp in the north-central region of Florida, the diet of seminole bats consisted of 90% Odonata and 10% Coleoptera during the month of July. Later in the summer, during the month of August, 90% of the diet was composed of Coleoptera and the remaining 10% being Hymenoptera (Zinn 1977).
Reproduction: The breeding season in eastern Texas commences during the fall and winter. The female retains the sperm in the uterus until spring, when ovulation and fertilization occur. Lasuirus seminolus produce a litter in late May or June. There are no records of pregnant females from eastern Texas of the seminole bat, but lactating females were collected in late June (Schmidly 1983).
Embryo counts for pregnant females in Florida ranged from one to four (Jennings 1958). Two pregnant females were collected in Putnam County, Florida. One weighed 20 grams and the other 16.9 grams. Stripped of their extra-embryonic membranes, the pair of embryos weighed 2.3 grams of their motherís gross 16.9 grams and the set of four weighed 6 grams of the motherís gross 20 grams. Another female lactating bat collected weighed 10.3 grams (Moore 1949). Pregnant females have been captured in Louisiana in early June. It is suspected that most pregnant females in Louisiana give birth to their young by the end of the second week of June (Lowery 1974)
Litter sizes range from one to four, (normally two). The large and well-developed baby bat is received in a pocket formed by the interfemoral membrane of the mother. The neo-nate crawls to the motherís breast and attaches to a nipple to nurse. The young are capable of flight after three to four weeks of nursing (Schmidly 1983).
Behavior: It has been noted in Alabama and South Carolina, that Seminole and red bats have been observed flying and feeding simultaneously in the same area. These two species occupied the same general habitats (Barkalow 1948; Coleman 1950). In the coastal plains of North Carolina, seminole bats were not seen along the coastal streams (Barkalow & Funderburg 1960). Observations reveal that Lasiurus seminolus and Lasiurus borealis feed rather early in the evening. Shortly after they take flight, the evening bat (Nycticeius humeralis) takes wing. Lastly, or just before dark, big brown bats (Eptesicus fuscus) and pipistrelles (Pipistrellus s. subflavus) take flight (Barkalow 1948)
Lasiurus seminolus is a solitary bat species (Lowery 1974). They are active during all seasons. In the Alabama coastal regions, seminole bats remain active by feeding regularly during the warmer days of winter (Barkalow 1948).
The two following sources provides evidence that seminole bats may not migrate, instead they remain in an area year around. From records taken in Charleston, South Carolina, it seems that Lasiurus seminolus is a permanent resident of this coastal region Coleman 1950). Similarly, since this species has been taken in east Texas from February through November, it is suggested that seminole bats are year long residents of this area (Schmidly et al. 1977).
Habitat: The ecological distribution of Lasiurus seminolus generally corresponds with that of Spanish moss (Tillandsia usneoides), in which these bats frequently roost (Barbour and Davis 1969; Constantine 1958; Dunaway 1960; Lowery 1974). Seminole bats may also roost beneath loose bark or in clumps of foliage other than Spanish moss (Sealander 1979). Because Lasiurus seminolus is a tree bat, it tends to inhabit wooded areas within its range (Laerm et al. 1980).
In Okefenokee Swamp, Georgia, seminole bats occur in the following habitats: uplands, prairies, islands, blackgum forest, pure cypress, mixed cypress, and pure bay forest (Laerm et al. 1980). Observations of this species were also made in moss-laden oak tree areas of southwestern Georgia. The bats roosted in a forest composed primarily of oak trees (Quercus virginiana, Quercus lyrata; Constantine 1958). In Florida, this species of bat inhabits hammocks, river swamps, scrubby flatwoods, pine flatwoods, and lowland forests (Ivey 1959; Jennings 1958; Moore 1949; Zinn 1977). Seminole bats collected in Tennessee inhabited forested areas containing the following tree species: oak, hickory (Carya sp.), beech (Betulaceae), dogwood (Cornus sp.), buckeye (Aesculus sp.), birch (Betula sp.), maple and hemlock (family uncertain; Kennedy et al. 1984).
The seminole bat is distributed throughout the Austroriparian Biotic Province of eastern Texas. Lasiurus seminolus is abundant throughout the pine-oak, long-leaf pine forests, and coastal prairie (Schmidly et al. 1977). It is noted that the seminole bat also, inhabits areas located in the Texan Biotic Province (Schmidly 1983). In the oak-hickory woodlands, this chiropteran is less common (Schmidly et al. 1977).
Economic Importance for Humans:
Like other insectivorous bats, the seminole bat is of economic assistance to man in that it exerts a check on the insect pest population (Dalquest and Horner 1984; Phillips 1966). There are very few reports of any parasitic organisms that may prey upon Lasiurus seminolus. Endoparasites and ectoparasites were not reported for seminole bats (Ubelaker 1970). Although mites (Acari) are known for Lasiurus borealis, Lasiurus cinereus, and Lasiurus intermedius, none were listed for seminole bats (Whitaker and Wilson 1974).
Studies done in Florida revealed that the seminole bat is a carrier of rabies. The prevalence of rabies in this species seems to be rare. Of the specimens collected in central Florida in 1953, only one of the sixty-one was infected with the rabies virus (Venters et al. 1954). From 1953-1956, 5 of the 785 bats inspected were positive for rabies (Schneider 1957). Bats may act as a reservoir of the rabies virus, but direct exposure of human beings to rabies by seminole bats in Florida or the United States is a minor hazard (Venters et al. 1954).
Of the bats captured in Georgia, defensive attempts to bite were made but no uprovoked attempts to bite were noted (Constantine 1958). They attack man only in the most unusual circumstances (Venters et al. 1954).
Conservation Status:
The categorical status of Lasiurus seminolus is considered common. This species is one that is abundant and widely distributed wherever it lives in a region (Schmidly 1983). Resting bats are exposed to predation, but are rarely preyed upon by other animals. In most instances, birds, such as the blue jay (Cyanocitta cristata) intrude upon them (Lowery 1974). Man is probably this chiropteranís greatest predator. Many of the seminole bats collected had numerous previous injuries. One of the most prevalent injuries observed were holes from shot (Constantine 1958). Another factor resulting in losses of the Seminole bat is commercial moss collecting, reducing the preferred roosting site of this species (Lowery 1974).
References:
Barbour, R. W. and W. H. Davis. 1969. Bats of America. University Press, Lexington Kentucky.
Barkalow, S. 1948. The status of the seminole bat, Lasiurus seminolus (Rhoads). Journal of Mammalogy 29:415-416.
Barkalow, F. S. AND J. B. Funderburg. 1960. Probable breeding and additional records of the seminole bat in North Carolina. Journal of Mammalogy 41:394-395.
Coleman, R. H. 1950. The status of Lasiurus borealis seminolus. Journal of Mammalogy 31:190-192.
Constantine, D. G. 1958. Ecological observations on lasiurine bats in Georgia. Journal of Mammalogy 39:64-70.
Dalquest, W. W. And N. V. Horner. 1984. Mammals of north-central Texas. Midwestern State University Press, Wichita Falls, Texas.
Dunaway, P. B. 1960. Seminole bat strangles by spanish moss. Journal of Mammalogy 41:400.
Hall, E. R. 1981. The mammals of North America. John Wiley and Sons Publications, New York.
Ivey, R. D. 1959. The mammals of Palm Valley, Florida. Journal of Mammalogy 40:585-586.
Jennings, W. L. 1958. The ecological distribution of bats in Florida. Ph.D. dissertation, University of Florida, Gainesville.
Kennedy, M. L., P. K. Kennedy AND G. D. Baumgardner. 1984. First record of the seminole bat (Lasiurus seminolus) in Tennessee. Journal Tennessee Academy of Science 59:89-90.
Laerm, J., B. J. Freeman, L. J. Vitt, J. M. Meyers, AND L. Logan. 1980. Vertebrates of the Okefenokee Swamp. Brimleyana 4:47-73.
Layne, J. N. 1955. Seminole bat, Lasiurus seminolus, in central New York. Journal of Mammalogy 36:453.
Lowery, G. H. 1974. The mammals of Louisiana and its adjacent waters. Louisiana State University Press, Baton Rouge.
Miller. G. S. Jr. 1897. Revision of the north american bats of the family Vespertilionidae. North American Fauna 13:1-135.
Moore, J. C. 1949. Putnam County and other Florida mammal notes. Journal of Mammalogy 30:57-66.
Phillips, G. L. 1966. Ecology of the big brown bat (Chiroptera: Vespertilionidae) in Northeastern Kansas. The American Midland Naturalist 75:168-198.
Poole, E. L. 1932. Lasiurus seminolus in Pennsylvania. Journal of Mammalogy 13:162.
Poole, E. L. 1949. A second Pennsylvania specimen of Lasiurus seminolus (Rhoads) Journal of Mammalogy 30:80.
Schmidly, D. J. 1983. Texas mammals east of the Balcones fault zone. Texas A&M University Press, College Station.
Schmidly, D. J., K. T. Wilkins, R. L. Honeycutt And B. C. Weynand. 1977. The bats of east Texas. Texas Journal of Science 28:127-143.
Schneider, N. J., J. E. Scatterday, A. L. Lewis, W. L. Jennings, H. D. Venters, AND A.V. Hardy. 1957. Rabies in bats in Florida. American Journal of Public Health 47:983-989.
Sealander, H. B. 1979. A guide to Arkansas mammals. River Road Press, Conway, Arkansas.
Sherman, H. B. 1939. Notes on the food of some Florida bats. Journal of Mammalogy 20:103-104.Ubelaker, J. E. 1970. Some observation on ecto- and endo- parasites of chiroptera. Pp. 247-261 in About bats: a chiropteran biology symposium (B. H. Slaughter and D. W. Walton, eds.). Southern Methodist University, Dallas, Texas.
Venters, H. D., W. R. Hoffert, J. E. Scatterday, And A. V. Hardy. 1954. Rabies in bats in Florida. American Journal of Public Health 44:182-185.
Whitaker, J. O., AND N. Wilson. 1974. Host and distribution lists of mites (Acari), parasitic and phoretic, and in the hair of wild mammals of north america, north of Mexico. American Midland Naturalist 91:1-67.
Zinn, T. L. 1977. Community ecology of Florida bats with emphasis on Myotis austroriparius. M.S. thesis, University of Florida, Gainesville.