 |
 Map prepared by Greg T. Lewellen |
Eptesicus fuscus (Big Brown Bat)
Written by
Theresa Albrecht (Mammalogy
Lab--Fall 2003)
Edited by Karah Gallagher and Jennifer Bailey
 |
 Map prepared by Greg T. Lewellen |
The range of the big brown bat begins as far north as Canada and extends southward to the northern portion of South America. Eptesicus fuscus is found throughout the United States as far south as the Carribbean islands (Barbour and Davis 1969). Eptesicus fuscus is also found in Central America and Mexico (Davis 1966). The big brown bat is abundant throughout much of the United States. Four subspecies are presently recognized in the United States. Eptesicus fuscus fuscus occurs east of the Great Plains except peninsular Florida where a darker race, Eptesicus fuscus osceola, is found. A pale race, Eptesicus fuscus pallidus is found from the western plains through the Rocky Mountains. In the Pacific states the more brightly colored Eptesicus fuscus bernardinus occurs (Barbour and Davis 1969). This species of bat is unknown in most of Alaska, in the southern portion of Florida, and much of south-central Texas (Barbour and Davis 1969; Dalquest and Horner 1984).
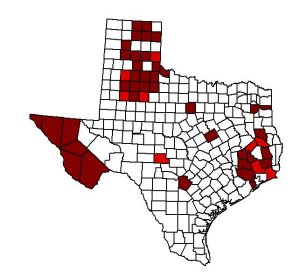
The largest concentration of Eptesicus fuscus occurs in the eastern section of the Texas panhandle. Records from north-central Texas are all from the cedar brakes on the west, apparently because of the gypsum caves there. Additional records of Eptesicus fuscus are from Hardeman county (Dalquest and Horner 1984). A portion of a lower jaw was found at the Coffee Ranch Quarry, 17 kilometers northeast of Miami, in Hemphill County. Given the location in which it was found and the similarity of the molars of modern Eptesicus, it was named Eptesicus hemphillensis (Dalquest 1983).
The subspecies Eptesicus fuscus pallidus has been documented in two counties in the southeastern corner of the Texas Panhandle. One specimen has been recorded in Childress county, 12 miles south of Childress. Another specimen was recorded in Hardeman county, 3 miles southeast of Lazare. Ten Eptesicus fuscus pallidus were examined from an area southwest of Quanah (Dalquest and Horner 1984).
Physical Characteristics: The big brown bat is most similar to the species Nycticeius humeralis from which it can be distinguished by its larger size. The big brown bat is a medium-sized bat in which its pelage color can vary from russet to dark brown. Big brown bats also possess a keeled calcar (Barbour and Davis 1969). The pelage of the adult big brown bat varies dorsally from chestnut-brown to sepia (Miller 1897; Phillips 1966). The underside also varies but is a paler brown. When compared to Eptesicus fuscus pallidus, the subspecies west of Eptesicus fuscus, the pelage is much paler. The pelage of adult big brown bats is long, glossy, and lax. Hairs on the face and along the edges of the membranes are shorter, more delicate, and paler than the hairs that cover the rest of the body. In younger big brown bats, the pelage is duller, darker, and shorter than in the adults. There is very little differentiation between the dorsal and ventral pelage color in the young bats. In Leavenworth, Kansas, the pelage of the underside varied greatly in comparison to the topside. Ventrally, the shades varied from cinnamon to buffy-brown, grayish, or silvery (Phillips 1966). A specimen from Brazos, Texas, was described as having light brown hair on its dorsal side and ventral sides and brown colored membranes (Allen 1894).
Eptesicus fuscus has a plain broad nose without appendages (Rafinesque 1820). The short, erect ears incline outward and are separated. The tragus is broad and rounded (Barbour and Davis 1969). The tip of the tragus is blunt (Allen 1894). The big brown batís long tail is inside its naked interfemoral membrane. The membranes are black and the wings are characterized as short and broad (Davis and Schmidly 1994).
Total length for Eptesicus fuscus averages from 106 to 127 millimeters (Phillips 1966). Approximate weights for this species range from 13 to 18 grams. Females tend to have a greater total length and wingspread than do males (Phillips 1966). The motherís forearm length averages around 45.8 millimeters, whereas the neonatal forearm length averages approximately 16.8 millimeters (Kurta and Kunz 1987).
External measurements of Eptesicus fuscus from north-central Texas between males and females differ. The average measurements for the male are: total length 112mm, length of tail 45.5mm, length of hindfoot 11.2mm, and height of ear 16.8mm. The average measurements for the female are: total length 119mm, length of tail 46.1mm, length of hind foot11.0mm, and height of ear 16.1mm (Dalquest and Horner 1984).
The typical skull of Eptesicus fuscus averages about 18.5 mm in occipital nasal length, and 12.5 mm in zygomatic breadth (Miller 1897). In Eptesicus fuscus, the dental formula is i 2/3 c 1/1 p 1/2 m 3/3 with a total of 32 teeth. Both upper incisors are well developed. The inner incisor is larger than the outer and usually with a distinct secondary cusp. The crown of the third lower incisor is wider than that of the first and second incisor. The incisors are closely crowded and the edges overlap forming a strong convex row between canines (Allen 1894; Miller 1907). Canine teeth are very sharp, curved, and long (Rafinesque 1820). The canines are simple with no secondary cusps. The premolars and first and second upper molars are normal with no special peculiarities. The third upper molar is variable in form. All the cusps of the lower molars are present and of normal form (Miller 1907).
Natural History:
Food Habits: Eptesicus fuscus is an insectivorous mammal that feeds primarily during the evening hours. The big brown bat emerges at dusk and pursues night-flying insects in cleared meadows, among the scattered trees in pastures, along tree-lined village streets, or above the traffic in the middle of a city (Barbour and Davis 1969). Fecal droppings examined in West Virginia during the month of August determined the following percentages of identifiable material by frequency of occurrence: Coleoptera (36.1%), Hymenoptera (26.3%), Diptera (13.2%), Plecoptera (6.5%), Ephemerida (4.6%), Hemiptera (3.4%), Trichoptera (3.2%), Neuroptera (3.2%), Mecoptera (2.7%), and Orthoptera (0.6%) (Hamilton 1933). Some common names of the insects consumed were June bugs, ground beetles, true flies, true bugs, stinkbugs, stone flies, caddisflies, flying ants, may flies, lace-wing flies, scorpion flies, and grasshoppers. The kinds of insects found in stomachs of big brown bats from Leavenworth, Kansas, by percentage of occurrence: Coleoptera (100%), Hemiptera (100%), Hymenoptera (40%), Diptera (30%), Homoptera (20%), and Lepidoptera (10%). Although, lepidopterans are a major part of the diet of many species of bats, butterflies and moths do not constitute a majority of the diet of the big brown bat (Phillips 1966).
It was estimated that Eptesicus fuscus may capture more than 600 insects per hour (Phillips 1966).
Eptesicus fuscus is a microchiropteran that locates, identifies, and captures prey by using a biological sonar system (Geggie and Fenton 1985; Griffen et al. 1960; Hamr and Bailey 1985; Poussin and Simmons 1982). Observational evidence suggests that bats listen passively to sounds produced by wing beats of insect prey or perhaps by other sounds emitted by insects and orient on these sounds. Recognition and discrimination of insect groups has usually been attributed to the acute properties of echolocation (Hamr and Bailey 1985). Results of experiments show that the sensitivity of hearing in the insectivorous, echolocating bat, Eptesicus fuscus, ranges in frequency from 200 Hz to 5kHz (Poussin and Simmons 1982).
Insect pursuit behavior by the big brown bat is distinguished by three phases. The search phase is characterized by straight flight. The second phase, or approach phase begins when the bat is ready for capturing its prey. The bat turns towards the insect and increases its repetitive pulse rate. During the terminal phase, the bat emits a burst of pulses at a very high rate and closes in on the insect (Griffin et al. 1960).
Reproduction: The big brown bat copulates in September and October. The spermatozoa are stored in the uterus during the winter and the sperm may fertilize ova released from the ovaries in the spring (Wimsatt 1945). For pregnant females, prolonged periods of torpor coincide with prolonged gestation (Audet and Fenton 1988). Ovulation is spontaneous and occurs late in April or early in May (Wimsatt 1945). This reproductive strategy allows gestation to begin immediately following emergence from hibernation in spring providing maximum time for juveniles to grow and deposit fat reserves before entering hibernation in autumn (Burnett and Kunz 1982). The uterus of Eptesicus fuscus is bilaterally symmetrical which allows the mother to carry more than one fetus (Wimsatt 1945). In eastern populations of the big brown bat, three to seven ova are typically shed, fertilized, and implanted, but only one in each horn survives to parturition (Birney and Baird 1985). In western North America, the majority of the ova implant in the right horn of the uterus. Consequently, the ovum in the left horn is reabsorbed. Fetal sex ratio is near 1 to 1 (Schowalter and Gunson 1979).
Females produce two young per litter in the eastern part of the range and one per litter in the western part (Cockrum 1955). Litter size in western North Dakota indicates that litter size is usually one (Jones and Genoways 1966). The average litter size in Iowa is two (Kunz 1974). In Kansas, the usual number per litter is two (Phillips 1966). Two young per female is the normal number in Kentucky (Davis et al. 1968).
Parturition times also vary geographically (Barbour and Davis 1969). Parturition in central Kansas occurs over a three-week period with most young born in the first week of June (Kunz 1974). In Maryland, parturition lasts for five weeks with most young born in late May (Christian 1956). In Kentucky, parturition begins in late May with births occurring in early June (Davis et al. 1968). The average neonatal body mass is 3.3 grams. The motherís postpartum body mass averages 16.1 grams (Kurta and Kunz 1987).
During weaning, the mothers leave the neonates behind to forage for only about one hour. Upon return, the female crawls over the colony till she finds her own. She licks them about their face and lips before allowing them to nurse (Barbour and Davis 1969; Davis et al. 1968). The mean lactation period lasts approximately forty days (Kunz 1974). During this time, the newborn clings to the motherís teats and are sheltered beneath her membranes during the day (Barbour and Davis 1969).
Bats are not well furred at birth and are incapable of thermoregulation. Despite their tenacious clinging ability, neonates are relatively helpless at birth and are totally dependent upon the mother (Kurta and Kunz 1987). On occasion, a neonate will fall to the floor of a nesting site. At night, mothers will retrieve their young upon recognition. Female big brown bats are also able to locate their young by the continual squeaking sounds of the fallen or strayed baby (Barbour and Davis 1969; Davis et al. 1968).
Behavior: Members of Eptesicus fuscus, are classified as good flyers based on distances traveled ranging from 3,360 to 4,540 millimeters per second (Craft et al. 1958). Flight speeds of the big brown bat in a maternity colony averaged at 20.8 miles per hour (Patterson and Fenton 1969). Eptesicus fuscus flies at levels estimated from 15 to 30 feet above the ground and at heights up to 50 feet above a river. The flight of foraging bats consists of circular movements with dips and rises incorporated in the pattern (Phillips 1966).
Eptesicus fuscus has a remarkable homing ability. Seven of the one hundred and fifty-five big brown bats captured and released returned home to Cincinnati, Ohio, from a distance of 450 miles (Smith and Goodpaster 1958).
The big brown bat is hardy (>20grams) and characteristically enters hibernation in late autumn (Beer and Richards 1956; Fenton 1972). They are poikilotherms, which means that their metabolism during this inactive state shows no evidence of a thermoregulatory mechanism. Hibernation is defined as a relatively long period in which the bat is subjected to low environmental temperatures, is inactive, and has a very low metabolic rate correlated with a low body temperature. Hibernation sites have comparatively low humidity, variable temperature, and exposure to air movements. In a cave near St. Paul, Minnesota, most of the hibernating bats were found in areas with little or no light with temperatures averaging around 42 degrees Fahrenheit (Beer and Richards 1956). Clusters consist mainly of the male sex, while females are more solitary. Throughout the winter, big brown bats may move from one cave to another during periods of arousal and activity (Phillips 1966). Eptesicus fuscus found active during winter months may be forced into activity because of reduced energy reserves. Under normal hibernation situations, the energy reserves are adequate. On the other hand, if hibernacula temperatures are too high or conditions are dry, inadequate reserves may arouse the bat during the winter in attempts to locate food (Brigham 1987).
In the spring, Eptesicus fuscus, emerges from torpor. Within ten minutes, the big brown bat spreads its wings, which is then followed by rapid shivering, and it is ready for flight (Phillips 1966). The average mass of bats leaving hibernation in April is approximately 16.4 grams (Fenton 1972).
Eptesicus fuscus in summer colonies are not tolerant of high temperatures. They are known to abandon a roosting spot as temperatures reach 92 degrees Fahrenheit (Barbour and Davis 1969; Davis et al. 1968).
Habitat: The big brown bat is a sedentary species (Davis et al. 1968). Both proximity to roosts and prey availability can influence the distribution of bats. Eptesicus fuscus favor roosts in buildings. During the summer months they roost in attics, barns, bridges, hollow trees, and rock crevices. In the winter, they can be found hanging in caves, tunnels, and other shelters (Barbour and Davis 1969). In northeastern Kansas, a limestone mine, near the city of Leavenworth houses the largest colony of the big brown bat in the state. The same species of bat was found roosting in a storm sewer in the city of Lawrence, Kansas (Phillips 1966). The big brown bat is a colonial species that is known to roost in artificial structures and commonly lives in urban environments. Although, urban habitats appear to provide Eptesicus fuscus with a wealth of roosting sites, food supplies are lower here (Geggie and Fenton 1985). Eptesicus fuscus does not restrict itself to specific habitat types during foraging. The big brown bat is the most flexible of many bat species in terms of habitats where it forages and its use of rich food patches in rural, town, or city settings. This particular species exhibits significant positive associations with lights. In fact, studies show that Eptesicus fuscus exploit concentrations of insects around lighted rural and urban areas (Furlonger et al. 1987).
Economic Importance for Humans:
The big brown bat is of economic assistance to man in that it exerts a check on the insect pest population (Dalquest and Horner 1984; Phillips 1966). Because of its closeness to the human population, we study them. Understanding how wildlife species respond to urbanization is interesting and studying them allows us to learn more about the batís basic ecology (Geggie and Fenton 1985).
Because the species does not typically dwell in caves, the big brown bat seems to be a nuisance as it chooses to roost inside human dwellings (Phillips 1966). Humans find them as a bit of an annoyance, as they enter houses. In addition to being very noisy, these bats deposit quantities of fecal pellets (Dalquest and Horner 1984). Big brown bats tend to show up in houses most commonly in August and September (Beer 1955).
Conservation Status:
The big brown bat is a relatively abundant and widely distributed mammal over North America (Barbour and Davis 1969; Burnett 1983). South America is much more richly endowed than North America with members of this genus. Because of the great diversity of forms or subspecies and the range of the big brown bat, it is considered to be almost cosmopolitan (Davis 1966). Because Eptesicus fuscus seems to prosper well near human populations, it is likely that the population and range of habitat of this species will remain quite stable.
If roosting opportunities limit populations of bats to some extent, it is predicted that species such as Eptesicus fuscus should thrive along the interface between urban and rural settings since here they can exploit high roost densities where food availability is less diminished by development (Geggie and Fenton 1985).
References:
Allen, H. 1894. A monograph of the bats of North America. Bulletin of the United States National Museum 43:1-198.
Audet, D., and M.B. Fenton. 1988. Heterothermy and the use of torpor by the bat Eptesicus fuscus (Chiroptera: Vespertilionidae): a field study. Physiological Zoology 61:197-204.
Barbour, R. W. and W. H. Davis. 1969. Bats of America. University Press, Lexington, Kentucky.
Beer, J. R. 1955. Survival and movements of banded big brown bats. Journal of Mammalogy 36:242-248.
Beer, J. R. and A. G. Richards. 1956. Hibernation of the big brown bat. Journal of Mammalogy 37:31-41.
Birney, E. C., and D. D. Baird. 1985. Why do some mammals polyovulate to produce a litter of two? The American Naturalist 126:136-140.
Brigham, R. M. 1987. The significance of winter activity by the big brown bat (Eptesicus fuscus): the influence of energy reserves. Canadian Journal of Zoology 65:1240-1242.
Burnett, C. D. 1983. Geographic and climatic correlates of morphological variation in Eptesicus fuscus. Journal of Mammology 64:437-444.
Burnett, C. D. and T. H. Kuntz. 1982. Growth rates and age estimation in Eptesicus fuscus and comparison with Myotis lucifugus. Journal of Mammalogy 63:33-41.
Christian, J. J. 1956. The natural history of a summer aggregation of the big brown bat Eptesicus fuscus fuscus. The American Midland Naturalist 55:66-95.
Cockrom, E. L. 1955. Reproduction of North American bats. Transactions of the Kansas Academy of Sciences 58:487-511.
Craft, T. J., M. I. Edmondson, and R. Agee. 1958. Comparative study of the mechanics of flying and swimming in some common brown bats. The Ohio Journal of Science 58:245-249.
Dalquest, W. W. 1983. Mammals of the Coffee Ranch Local Fauna Hemphillian of Texas. Texas Memorial Museum, University of Texas, Austin 38:8-9.
Dalquest, W. W. And N. V. Horner. 1984. Mammals of North-Central Texas. Midwestern State University Press, Wichita Falls, Texas.
Davis, W. B. 1966. Review of South American Bats of the Genus Eptesicus. The Southwestern Naturalist 11:245-274.
Davis, W. B. And D. J. Schmidly. 1994. The mammals of Texas. Texas Parks And Wildlife Press, Austin.
Davis, W. H., R. W. Barbour, and M. D. Hassell. 1968. Colonial behavior of Eptesicus fuscus. Journal of Mammalogy 49:44-50.
Fenton, M. B. 1972. Distribution and overwintering of Myotis le Eptesicus fuscus (Chiroptera: Vespertilionidae) in Ontario. Museum of Life Science 21:1-18. Jones, J. K. And H. H. Genoways. 1966. Records of bats from western North Dakota. Transactions of the Kansas Academy of Sciences 69:88-90.
Furlonger, C. L., H. J. Dewar, and M. B. Fenton. 1987. Habitat use by foraging insectivorous bats. Canadian Journal of Zoology 65:284-288.
Geggie, J., and M. B. Fenton. 1985. A comparison of foraging by Eptesicus fuscus (Chiroptera: Vespertilionidae) in urban and rural environments. Canadian Journal of Zoology 63:263-267.
Griffin, D. R., F. A. Webster, and C. R. Michael. 1960. The ecolocation of flying insects by bats. Animal Behavior 8:141-154.
Hamilton, W. J. Jr. 1933. The insect food of the big brown food. Journal of mammalogy 14:155-156.
Hamr, J., and E. D. Bailey. 1985. Detection and discrimination of insect flight sounds by big brown bats (Eptesicus fuscus). Biology of Behaviour 10:105-121.
Hoff, G. L., and W. J. Bigler. 1981. The role of bats in the propagation and spread of histoplasmosis: a review. Journal of Wildlife Diseases 17:191-195.
Kunz, T. H. 1974. Reproduction, growth, and mortality of the vespertilionid bat, Eptesicus fuscus, in Kansas. Journal of Mammalogy 55:1-13.
Kurta, A., T. and T. H. Kunz. 1987. Size of bats and maternal investment during pregnancy. Symposium of the Zoological Society of London 57:79-106.
Miller. G. S. Jr. 1897. Revision of the North American bats of the family Vespertilionidae. North American Fauna 13:1-135.
Miller, G. S. Jr. 1907. The families and genera of bats. Bulletin of the United States Museum, Washington 57:1-282.
Moore, G. J., and G. H. Raymond. 1970. Prolonged incubation period of rabies in a naturally infected bat Eptesicus fuscus (Beauvois). The Journal of Wildlife diseases 6:167-168.
Patterson, A. P., and M. B. Fenton. 1969. Flight speeds of five species of bats. Journal of Mammalogy 50:152-153.
Phillips, G. L. 1966. Ecology of the big brown bat (Chiroptera: Vespertilionidae) in Northeastern Kansas. The American Midland Naturalist 75:168-198.
Poussin, C. and J. A. Simmons. 1982. Low-frequency hearing sensitivity in the echolocating bat, Eptesicus fuscus. Journal of the Acoustal Society of America 72:340-342.
Pybus, M. J. 1986. Rabies in insectivorous bats of western Canada, 1979-1983. The Journal of Wildlife Diseases 22:307-313.
Rafinesque, C. S. 1820. Annals of nature or annual synopsis of new genera and species of animals, plants, and c. discovered in North America. Thomas Smith, Lexington, Kentucky.
Schowalter, D. B., and J. R. Gunson. 1979. Reproductive biology of the big brown bat (Eptesicus fuscus) in Alberta. Canadian Field-Naturalist 93:48-54.
Smith, E. And W. Goodpastor. 1958. Homing in nonmigratory bats. Science 127:644 Wimsatt, W. A. 1945. Notes on breeding behavior, pregnancy, and parturition in some vespertilioid bats of the eastern United States. Journal of Mammalogy 26:23-33.